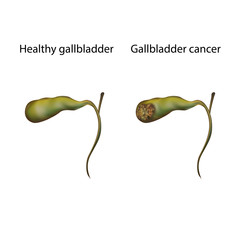
Before moving to the medications and latest developments, let's look a little bit at "What is gallbladder cancer?"
Gallbladder cancer is the mutation and uncontrollable growth of carcinogenic cells in the gallbladder. The unpredictable changes or mutations in the DNA (deoxyribonucleic acid) lead to a tumor. The cells grow out of control, which starts to pressure other healthy cells making them die and take their place. Even after that, the cancer cells do not stop, multiply, and begin to spread, disturbing the other organ's function. They affect the whole area where they get spread.
Gallbladder cancer starts in the epithelial cells that line the internal or inner surface of the organ. This type of cancer is not easily recognizable at the very first stage, so the chances increase as it becomes more extensive.
Now is the time to stay up to date on the most recent FDA-approved medications; keep reading to learn more!!
Take charge of your health and your life. Contact us today!
Can Gallbladder Cancer treated by FDA-Approved Durvalumab?
Durvalumab (Imfinzi, AstraZeneca UK Limited) was approved by the Food and Drug Administration on September 2, 2022, in combination with gemcitabine and cisplatin (BTC).
Research has shown that durvalumab can slow tumor growth and shrink tumors in some people with cancer. Previous studies of combining durvalumab and chemotherapy showed that this combination is active in advanced mesothelioma.
These modified exploratory findings indicated that durvalumab following chemoradiotherapy provides long-term PFS and OS advantages. At 4 years, an estimated 49.6% of durvalumab-treated patients are still alive (placebo, 36.3%), and 35.3% are still living and progression-free (placebo, 19.5%).
Note- The information provided above is based on medical knowledge and has been approved. However, it is advisable to consult your doctor for more details and personalized advice regarding your specific health concerns.
Your well-being is our priority - call us to book your appointment today
FAQs

What is the new FDA-approved treatment for gallbladder cancer?
Answer: The new FDA-approved treatment for gallbladder cancer could vary depending on the specific drug or therapy that has received approval. It is essential to refer to the FDA's official website or consult with healthcare professionals for the most up-to-date information on the latest approved treatments.
How does the newly approved treatment differ from existing treatment options?
Answer: The difference between the newly approved treatment and existing options could lie in its mechanism of action, efficacy, safety profile, or targeted patient population. Clinical studies and data supporting the FDA approval would provide more insights into these differences.
What stage of gallbladder cancer is the new treatment intended for?
Answer: The FDA approval for the new treatment might specify the stage of gallbladder cancer for which it is intended. Some treatments might be approved for early-stage cases, while others could be targeted at advanced or metastatic gallbladder cancer.
What are the potential benefits and risks associated with the FDA-approved treatment?
Answer: The potential benefits of the FDA-approved treatment may include improved survival rates, tumor shrinkage, or symptom relief. The risks could involve side effects, potential adverse reactions, or interactions with other medications. Patients should discuss these aspects with their healthcare providers before starting the treatment.
How can patients access the new treatment? Is it covered by insurance? Answer: Patients can typically access FDA-approved treatments through their healthcare providers or medical facilities. Insurance coverage for the new treatment would depend on individual insurance plans and the specific treatment's inclusion in their formulary.
Are there any specific eligibility criteria for receiving the FDA-approved treatment?
Answer: The FDA-approved treatment might have specific eligibility criteria, such as disease stage, previous treatments received, and overall health condition. Oncologists and healthcare providers would assess patients to determine if they meet these criteria.
What clinical trials and studies were conducted to support the FDA approval? Answer: To gain FDA approval, a new treatment must undergo rigorous clinical trials and studies to demonstrate its safety and efficacy. These trial results are submitted to the FDA for evaluation before approval is granted.
Are there any ongoing research or future developments related to gallbladder cancer treatment?
Answer: Medical research is continually progressing, and ongoing studies are exploring new treatment options and approaches for gallbladder cancer. Patients and healthcare providers can stay informed about the latest developments through medical journals, conferences, and reputable healthcare organizations.