Ulcerative Colitis is an inflammatory disease that causes inflammation and ulcers in your digestive tract.
It is a common disease, mostly in the US. Every one in 250 people in North America and Europe has an inflammatory bowel disease.
Ulcerative Colitis can be burdening and can sometimes lead to life-threatening complications.
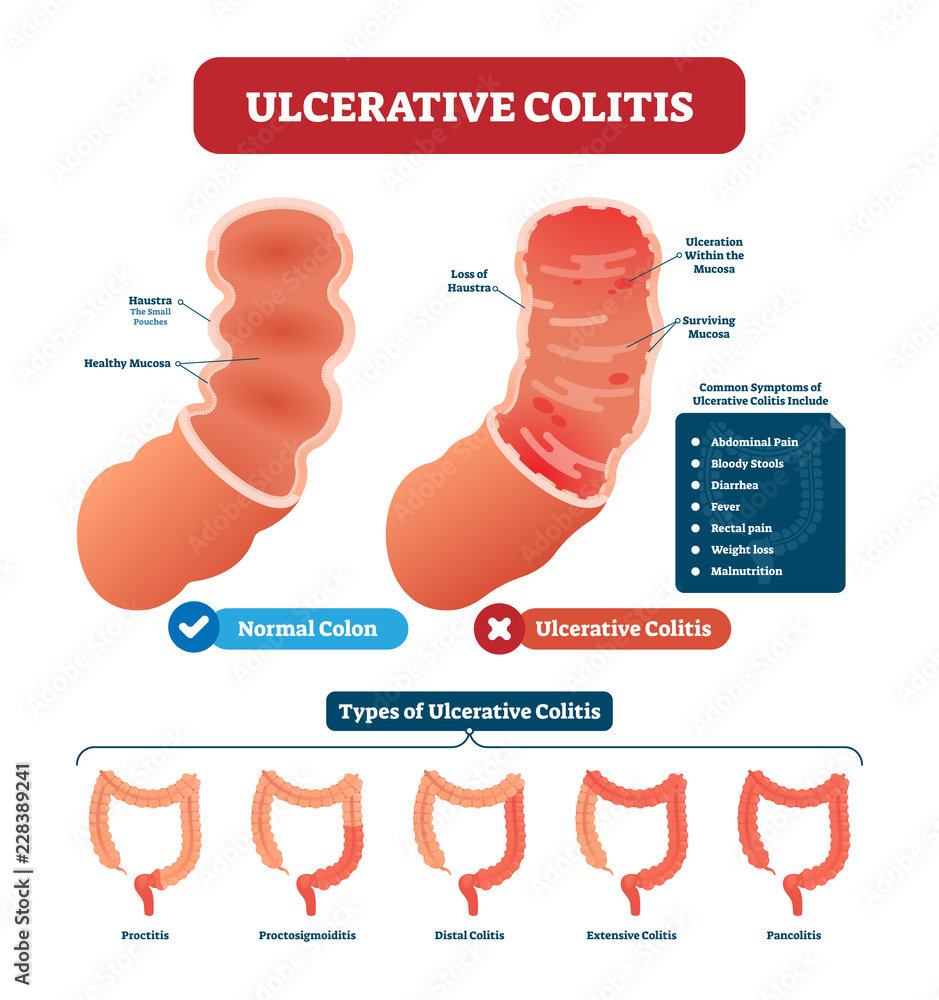
While it has no known cure, several new treatments can significantly reduce the signs and symptoms of the disease and bring about long-term remission.
More about New Treatment for Ulcerative Colitis
RINVOQ is the latest treatment for adults for moderately to severe ulcerative colitis, approved by the FDA on 16th March 2022. This medication is used to treat adults when one or more medicines called tumor necrosis factor (TNF) blockers are used and do not work well or cannot be tolerated.
Thomas Hudson, MD, senior vice president of research and development, and chief scientific officer of AbbVie, said, "With the approval of RINVOQ as a new treatment option, AbbVie continues our leadership in advancing research that can help impact the lives of people living with ulcerative colitis."
RINVOQ stabilizes natural proteins in our body called Janus Kinase (JAK) to reduce inflammation.
The safety and efficacy of RINVOQ in pediatric patients with Ulcerative Colitis are still unknown.
Does this new ulcerative colitis treatment have any side effects?
Like any other drug, RINVOQ too has adverse effects. No matter how insignificant, it is vital to know the side effects of the drug before taking it.
We present to you the side effects of Rinvoq, so read them carefully!
Note: RINVOQ is the latest ulcerative colitis treatment approved by the FDA. The above mentioned are only the known side effects of it as of now. There might be more adverse effects yet to be recognized.
There are many other factors you should know before considering RINVOQ.
We have discussed them below!
What do patients need to know before taking Rinvoq?
- RINVOQ may lower the body’s strength to fight infections. Some cases include tuberculosis and infections caused by fungi, bacteria, or contagious viruses.
- RINVOQ can increase the chances of cancer. Skin cancers and Lymphoma may occur. Patients with the habit of smoking are at higher risk of lung cancer.
- Increased risk of major cardiovascular issues, such as stroke, heart attack, or death, in people more than 50 years of age and at least 1 heart disease risk factor, especially if you have a habit of smoking.
- RINVOQ may cause blood clots in the veins of the lungs or legs and arteries.
- Symptoms such as rashes, difficulty breathing, dizziness, or swelling of your tongue, lips, or throat might mean you are allergic to an ingredient of RINVOQ. If these symptoms occur during treatment, discontinue RINVOQ and seek emergency medical help immediately.
- Your doctor should take regular blood tests before taking RINVOQ and while taking it.
Note: The Rinviq medication dosage your doctor prescribes depends on several factors, including the frequency and severity of your problem and other health conditions. Taking any medication requires the advice of a doctor.
References: